Sago Palm Studies
Sago Seed Germination
(Compiled from The Sago Palm, Kyoto Univ. Press, p99-104, 2015 *)
【Optimal temperature】
The optimum temperature for germination appears to be around 30 ˚C.
・The germination rate at 25 ˚C was not more than 20% (40 days after sowing (DAS)).
・The rate improved to about 40% (10–40 DAS) when the temperature was subsequently increased to 30 ˚C.
・As the 40-day germination rate at 35 ˚C was about 20%.
(see Table 2 in Ehara et al. 1998)
( [Source: Ehara, H., Komada, C. and Morita, O. (1998) Germination characteristics of sago palm and spine
emergence in seedlings produced from spineless palm seeds. Principes 42(4): 212–217.
https://palms.org/wp-content/uploads/2016/05/vol42n4p212-217.pdf]
【Physical treatment needed prior to sowing】
The removal of the exocarp, mesocarp and sarcotesta is
considered effective as physical treatment prior to seeding
(see below about the seed anatomy).
・There was no germination at all when the exocarp,
mesocarp and sarcotesta were all present together with seed
(under the condition of immersion in beakers of water
together with the removed tissues).
・The germination rate was 30% when only the exocarp and
mesocarp were present, and it was practically unhindered
in the presence of the sarcotesta alone.
・The removal of seed husk tissues can lead to germination
rates over 90%.
(see Figure 1, 2 and 4 in Ehara et al. 2001)
[Source: Ehara, H., Morita, O., Komada, C. and
Goto, M. (2001) Effect of physical treatment
and presence of the ricarp and sarcotesta on
seed germination in sago palm (Metroxylon
sagu Rottb.). Seed Science and Technology 29
(1): 83–90]
[* Ehara, H. (2015) Germination. In: The Society of
Sago Palm Studies ed., The Sago Palm: The Food
and Environmental Challenges of the 21st Century,
Kyoto University Press (Kyoto) and Trans Pacific
Press (Melbourne). p99–104, ISBN 978–1–920901
–13–4]
Seed Anatomy and Germination Aspect of Sago Palm
(Compiled from Sago Palm 14(2): 38−41, 2006**)
Seed anatomy
In sago palms (Metroxylon sagu Rottb.), flower buds are formed at the top of the tree crown (inflorescence) about 12 years after planting, and flowering occurs about two years after that (Yamamoto 1998). It takes at least three years from flower bud formation to fruit maturity (Flach 1997). Jong (1995) reported that that a single inflorescence can bear 1,313 to 3,427 rachillae (third-order branches). Although not all rachillae bear fruit, each rachilla often bears two or so fruits, meaning that one mother palm can bear around one thousand fruits, or in some cases several thousand.
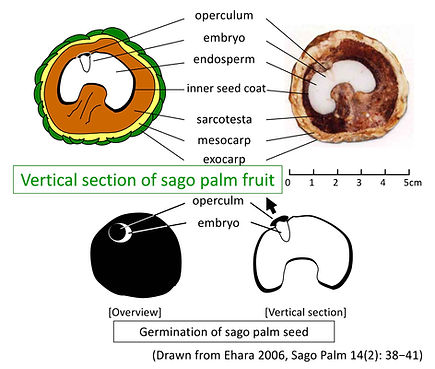
Sago palm seeds are albuminous seeds that contain an endosperm. The nutrient reserve that makes up the endosperm is cellulose, which becomes extremely hard when mature (Fig. 1 **). In this way, endosperm that becomes as hard as animal horn or ivory when mature is called horny endosperm (Werner 1999). The hardness of sago palm seeds is 400 kgf in the polar direction (vertical direction) (measured in a seed with an equatorial major axis of 2.7 cm and a polar diameter of 2.1 cm). The hardness of walnuts and pickled plum seeds is 20-50 kgf (a fruit with an equatorial major axis of 3.5 cm and a polar diameter of 4.3 cm) and 30-80 kgf (a seed with an equatorial major axis of 1.8 cm and a polar diameter of 2.3 cm), respectively.
The genus Metroxylon includes the section Metroxylon (section Eumetroxylon) (one species in one section), which includes the sago palm, as well as the section Coelococcus. Species that are classified into this section, such as the Pacific ivory palm (M. amicarum (H. Wendl.) Becc.: found in Chuuk and Pohnpei in Micronesia, and the Marshall Islands) and M. warburgii (Heim) Becc. (found in Vanuatu and Fiji in Melanesia, and Samoa in Polynesia), have seeds that are large in diameter (approximately 9 cm and 6 cm, respectively, in the equatorial axis of the fruit), and are used for buttons and crafts, known as palm ivory (Dowe 1989, Ehara et al. 2003, McClatchey 2006).
The embryo is embedded in the endosperm, and on the outside there is a lid-like organ called the operculum (Fig. 1 **). These are further covered by the seed coat tissues, namely the testa and the pericarp. The seed coat consists of two layers, a thin inner seed coat (black when mature) and a juicy fleshy sarcotesta on the outside. The pericarp consists of two layers, the mesocarp and exocarp, and the outermost exocarp is scale-like and arranged in 18 series of vertical rows.
In section Coelococcus, the number of scale rows varies by species, with 21-31 series, and some variations are reported to includde variation (24-29 series have been reported, but recent studies showed the above results: details will be given in another part in this article). The fruit is attached to the pedicel at the proximal end (Fig. 2 **). The size of the mature fruit is just over 5 cm in equatorial major axis, just under 5 cm in minor axis, and approximately 5 cm in polar diameter, with a fresh weight of about 50-60 g.
Seed germination process
Germination rates of sago palm seeds are generally low (Alang and Krishnapility 1986, Flach 1984, Jaman 1985, Johnson and Raymond 1956, Jong 1995, van Kraalingen 1984). When the seeds were sown in water at a temperature of 30°C, germination usually took 10 to 40 days, although in rare cases seeds took a year to germinate (Ehara et al. 1998, 2001).
Fig. 3 (**) shows a schematic of the germination state, and Figure 4 shows a typical germination process. Although the number of days until germination differs from individual to individual, the development of each organ after germination was regular (Ehara et al. 1998). (1) Germination is recognized when the embryo grows and pushes up the capsule. (2) About 6 days after germination, a coleorhiza-like organ appears. (3) This is followed by the epiblast, which begins to elongate 2 to 3 days later. (4) The primary root is extracted during the elongation of the epiblast (related to the timing when the capsule is pushed aside). (5) Two to three days after the appearance of the primary root, the first adventitious root appears from above the base of the coleorhiza-like organ (the base of the epiblast), and in sync with this, the coleoptile is extracted. (6) After another 2 to 3 days, the second adventitious root appears, and the first leaf appears on the same day. (7) At the same time that the second adventitious root elongates and the third root appears, branch roots develop from the main root. The number of days to germination and the germination rate are affected by sowing conditions such as the physical treatment of the seeds and the bed environment, but these will be discussed in detail in later sections.
References
Alang, Z. C. and B. Kirshnapillay 1986 Studies on the growth and development of embryos of the sago palms (Metroxylon spp. ) in vivo and in vitro. Sago-’85 Proc. Third Int. Sago Symposium (Tokyo) 121-129.
Dowe. J. L. 1989 Palms of the South-West Pacific: their origin, distribution and description. In: Palms of the South-West Pacific. (Dowe, J. L. ed.) Palms and Cycad Societies of Australia (Milton) 1-154.
Ehara, H., C. Komada and O. Morita 1998 Germination characteristics of sago palm seeds and spine emergence in seedlings produced from spineless palm seeds. Principes 42: 212-217.
https://palms.org/wp-content/uploads/2016/05/vol42n4p212-217.pdf
Ehara. H., O. Morita, C. Komada and M. Goto 2001 Effect of physical treatment and presence of the pericarp and sarcotesta on seed germination in sago palm (Metroxylon sagu Rottb.). Seed Sci. & Technol. 29: 83-90.
Ehara, H., H. Naito, C. Mizota and P. Ala 2003 Distribution, growth environment and utilization of Metroxylon palms in Vanuatu. Sago Palm 10: 64 - 72.
https://doi.org/10.57418/sagopalm.11.1_14
Flach, M. 1984 The sago palm. FAO Plant Production and Protection Paper 47. AGPC/MIS/80. FAO (Rome) pp.85.
Flach, M. 1997 Sago palm Metroxylon sagu Rottb.. International Plant Genetic Resources Institute (Rome) pp 76.
Jaman, O. H. 1985 The study of sago seed germination. Proc. 22nd Research Officers’ Conference, Kuching. Department of Agriculture, Sarawak, Malaysia. 69-78.
Johnson, R. M. and W. D. Raymond 1956 Sources of starch in colonial territories: Ⅰ: The sago palm. Colonial Plant and Animal Products 6: 20-32.
Jong, F. S. 1995 Germination of sago palm seeds. In: Research for the development of sago palm (Metroxylon sagu Rottb.) cultivation in Sarawak, Malaysia. Dr. Thesis of Agricultural University, Wageningen, The Netherlands. 120-130.
McClatchey, W. C., H. I. Manner and C. R. Elevitch 2006 Metroxylon amicarum, M. paulcoxii, M. sagu, M. salomonense, M. vitiense, and, M. warburgii (sago palm), Arecaceae (palm family). Species Profiles for Pacific Island Agroforestry, www.traditionaltree.org, April 2006 ver. (http://www.agroforestry.net/tti/Metroxylonsagopalm.pdf).
van. Kraalingen, D. W. G. 1984 Some observation on sago palm growth in Sepik River Basin, Papua New Guinea. Report of Department of Minerals and Energy, Konedobu, Papua New Guinea. pp.69.
[** Ehara, H. (2006) Seed Anatomy and Germination Aspect of Sago Palm. Sago Palm 14(2): 38−41
https://doi.org/10.57418/sagopalm.14.1_38]
[Fig.1**: see figure ② above on this site]
[Fig.2**: see figure ① above on this site]
[Fig.3**: see figure ② above on this site]
List of Publications Relating to Sago Palm Flower, Fruit, Seed and Germination
Ehara, H. (2015. Germination. In: The Society of Sago Palm Studies; Yamamoto, Y., Ehara, H. et al. eds., The Sago Palm: The Food and Environmental Challenges of the 21st Century. Kyoto University Press (Kyoto) and Trans Pacific Press (Melbourne). p99–104. ISBN 978–1–920901–13–4
Ehara, H., Naito, H., Tarimo, A. J. P., Bintoro, M. H. and Takamura, T. Y. (2006). Introduction of sago palm seeds and seedlings into Tanzania. Sago Palm 14(2): 65–71. https://doi.org/10.57418/sagopalm.14.2_65
Ehara H., Harley, M. M., Baker, W. J., Dransfield, J., Naito, H. and Mizota, C. (2006). Flower and pollen morphology of spiny and spineless sago palm in Indonesia. Japanese Journal of Tropical Agriculture 50(3): 121–126. https://doi.org/10.11248/jsta1957.50.121
Ehara, H. (2006). Seed Anatomy and Germination Aspect of Sago Palm. Sago Palm 14(1): 38-41. https://doi.org/10.57418/sagopalm.14.1_38
Ehara, H., Naito, H., Mizota, C. and Ala, P. (2003). Distribution, growth environment and utilization of Metroxylon palms in Vanuatu. Sago Palm 10(2): 64–72. https://doi.org/10.57418/sagopalm.10.2_64
Ehara, H., Morita, O., Komada, C. and Goto, M. (2001). Effect of physical treatment and presence of the pericarp and sarcotesta on seed germination in sago palm (Metroxylon sagu Rottb.). Seed Science and Technology 29(1): 83–90.
Ehara, H., Komada, C. and Morita, O. (1998). Germination characteristics of sago palm and spine emergence in seedlings produced from spineless palm seeds. Principes 42(4): 212–217. https://palms.org/wp-content/uploads/2016/05/vol42n4p212-217.pdf


① ②
③
④
